|
Polycyclic aromatic hydrocarbons (PAHs) and related aromatic materials are thought to be present in virtually all phases of the ISM (Allamandola, Hudgins, & Sandford 1999). Interstellar PAHs are most easily seen under conditions where gas phase PAHs (both neutrals and ions) are excited by UV and visible light and cool through the emission of infrared (IR) photons, giving rise to the well-known emission spectra with prominent features at 3050, 1610, 1300, 1160 and 890 cm-1 (3.28, 6.2, 7.6, 8.6, and 11.2 microns) (e.g., Allamandola, Tielens, & Barker 1989; Peeters et al. 2002). However, PAHs in dense interstellar clouds will be largely screened from exciting radiation. In addition, since dense molecular clouds are extremely cold (T < 50 K), most PAHs in these environments should be efficiently condensed onto dust grains, either as 'pure' solids or as 'guest molecules' in icy grain mantles, as is the case for most other interstellar molecules (Sandford & Allamandola 1993). The presence of PAHs in dense clouds is further suggested by the presence of aromatics in primitive meteorites and interplanetary dust particles that contain deuterium enrichments best explained by an interstellar dense cloud chemistry (Sandford 2002). Under dense cloud conditions, these PAHs will be seen in absorption.
Because they fall at positions characteristic of aromatic molecules, a few weak mid-infrared absorption features have been attributed to PAHs along lines of sight to a limited number of objects embedded in dense clouds. These include bands near 3030 cm-1 (3.3 microns) (Smith, Sellgren, & Tokunaga 1989; Sellgren et al. 1995; Brooke, Sellgren, & Geballe, 1999), and 1600 cm-1 (6.2 microns) (Chiar et al. 2000), and 890 cm-1 (11.2 microns) (Bregman, Hayward, & Sloan 2000).
Beyond making a general connection with aromatic materials, it has proven difficult to fully interpret these features. Although there is a growing database of laboratory IR absorption spectra of both neutral and ionized PAHs in inert gas matrices (e.g., Hudgins & Allamandola 1995a,b; Szczepanski, Wehlburg, & Vala 1995; Hudgins & Sandford 1998a,b,c; Mattioda et al. 2003; and references therein; see the PAHs in argon ice database), these data have been taken to address the interpretation of the IR emission bands of interstellar gas phase PAHs. However, the PAHs in dense clouds are expected to be condensed onto dust grains, largely as neutral molecules frozen in H2O rich ice mantles. Under these conditions, the PAHs will interact with each other and with other molecules. Because these interactions will perturb their infrared spectral properties (band positions, widths, profiles, and strengths), analyses of aromatic absorption bands in the spectra of dense clouds will require laboratory absorption spectra of appropriate analog materials taken under realistic astrophysical conditions.
Lines of sight that probe dense molecular clouds show that H2O is the dominant ice component present (Sandford 1996; Ehrenfreund & Charnley 2000; van Dishoeck 2004). Thus, for PAHs in dense clouds, the most important molecular interaction to consider is that between PAHs and H2O. H2O is a relatively polar molecule and can interact quite strongly with other molecules in the solid state (see, for example, Sandford et al. 1988; Ehrenfreund et al. 1998; Dartois et al. 1999; Gerakines et al. 1999). A systematic study of the infrared spectral properties of the two ring PAH naphthalene (C10H8) demonstrated that interactions with H2O resulted in relatively small changes in the positions and strengths of this molecule's vibrational bands, but significant changes in band widths (Sandford, Bernstein, & Allamandola 2004). However, to date, very little work has been done on the infrared spectral properties of multi-ring PAHs frozen in H2O-rich ices.
On this web page, we present the infrared spectra of 11 additional PAHs and related species frozen in H2O ices. In general the positions of PAH absorptions in H2O are not very different from those of PAHs in argon matrix.

Figure 1. The 4000-500 cm-1 (2.5-20 microns) infrared spectra of pyrene (C16H10) isolated in an argon matrix at 12 K (Ar/C16H10 > 1000; from Hudgins & Sandford 1998a) (top) and in an H2O matrix (H2O/C16H10~ 10) at 15 K (bottom). The H2O-pyrene spectrum is dominated by the H2O features at 3320, 1630, and 750 cm-1 (3.0, 6.1, and 13.3 microns), but also shows features associated with the fundamental vibrations of pyrene and an enhanced dangling O-H band near
3620 cm-1 (2.76 microns).
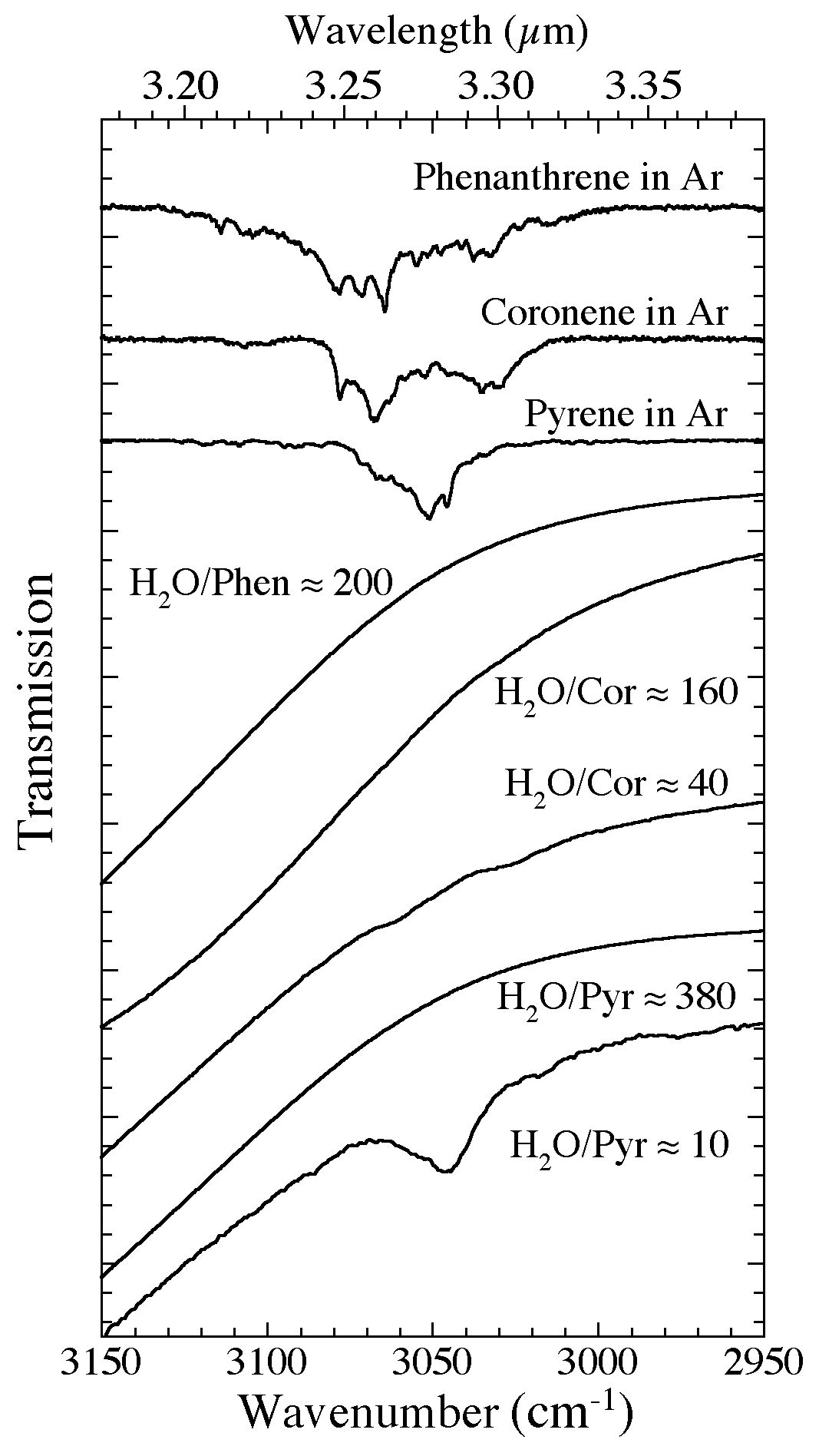
Figure 2. The 3150-2950 cm-1 (3.17-3.39 microns) spectra of phenanthrene, coronene, and pyrene in Ar and H2O matrices. The H2O-PAH spectra span a range of PAH concentrations and demonstrate how the presence of the nearby H2O band suppresses the spectral contrast of these vibrational modes. As with naphthalene, the C-H stretching bands of other PAHs only become weakly visible as the H2O/PAH ratio falls below ~50, i.e., PAH concentrations in the H2O exceed a few percent.
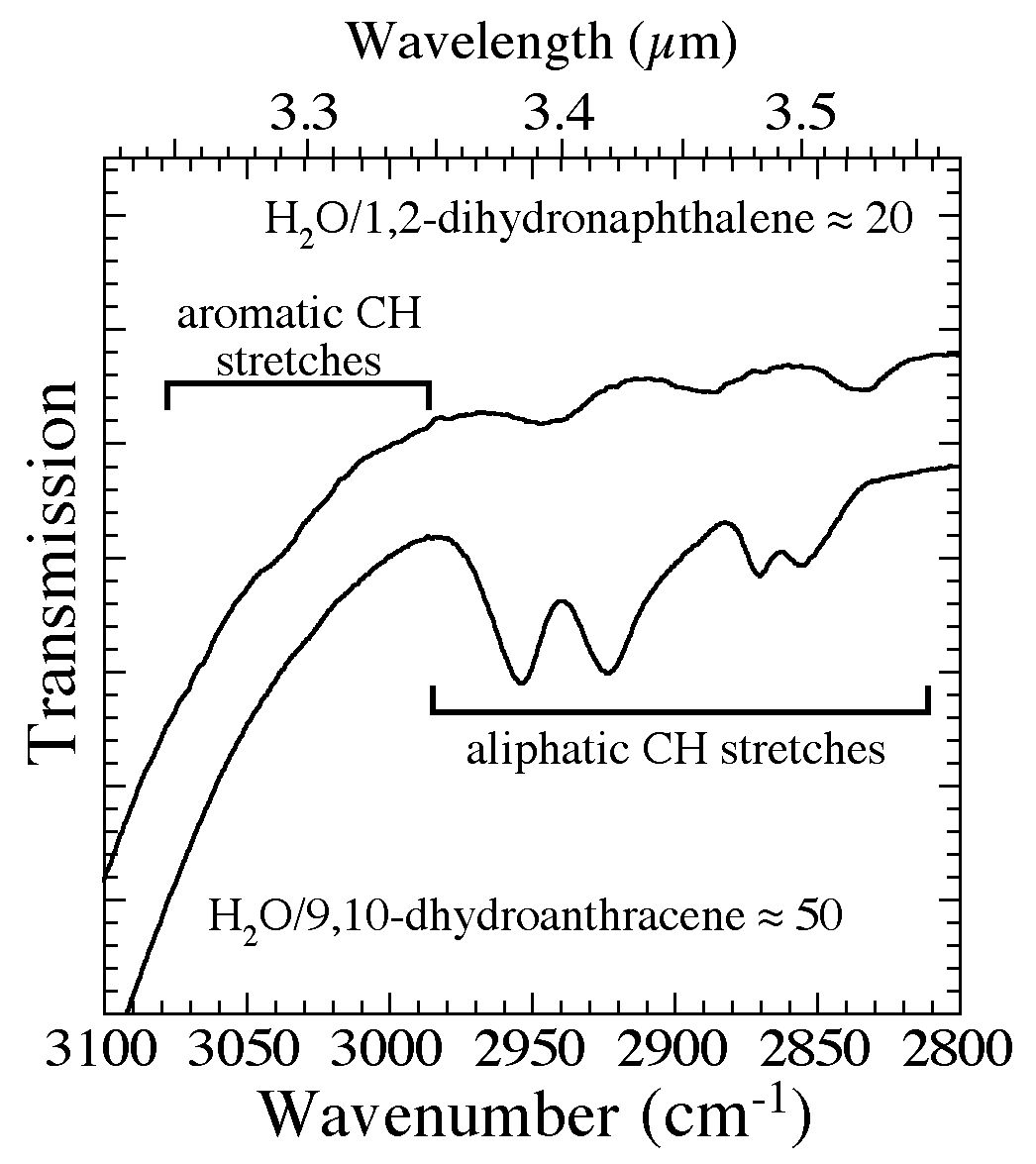
Figure 3. The 3100-2800 cm-1 (3.23-3.57 microns) spectra of 1,2-dihydronaphthalene and 9,10-dihydroanthracene in H2O
matrices (H2O/1,2-dihydronaphthalene ~ 20 and H2O/9,10-dihydroanthracene ~ 50). The structures for these
two molecules can be seen in Figures 4b and 4d. The regions in which aromatic and aliphatic CH stretching vibrations
fall are marked with horizontal bars. While the aromatic CH stretches bands of these molecule are barely visible at these concentrations, the aliphatic CH stretching bands are readily apparent.
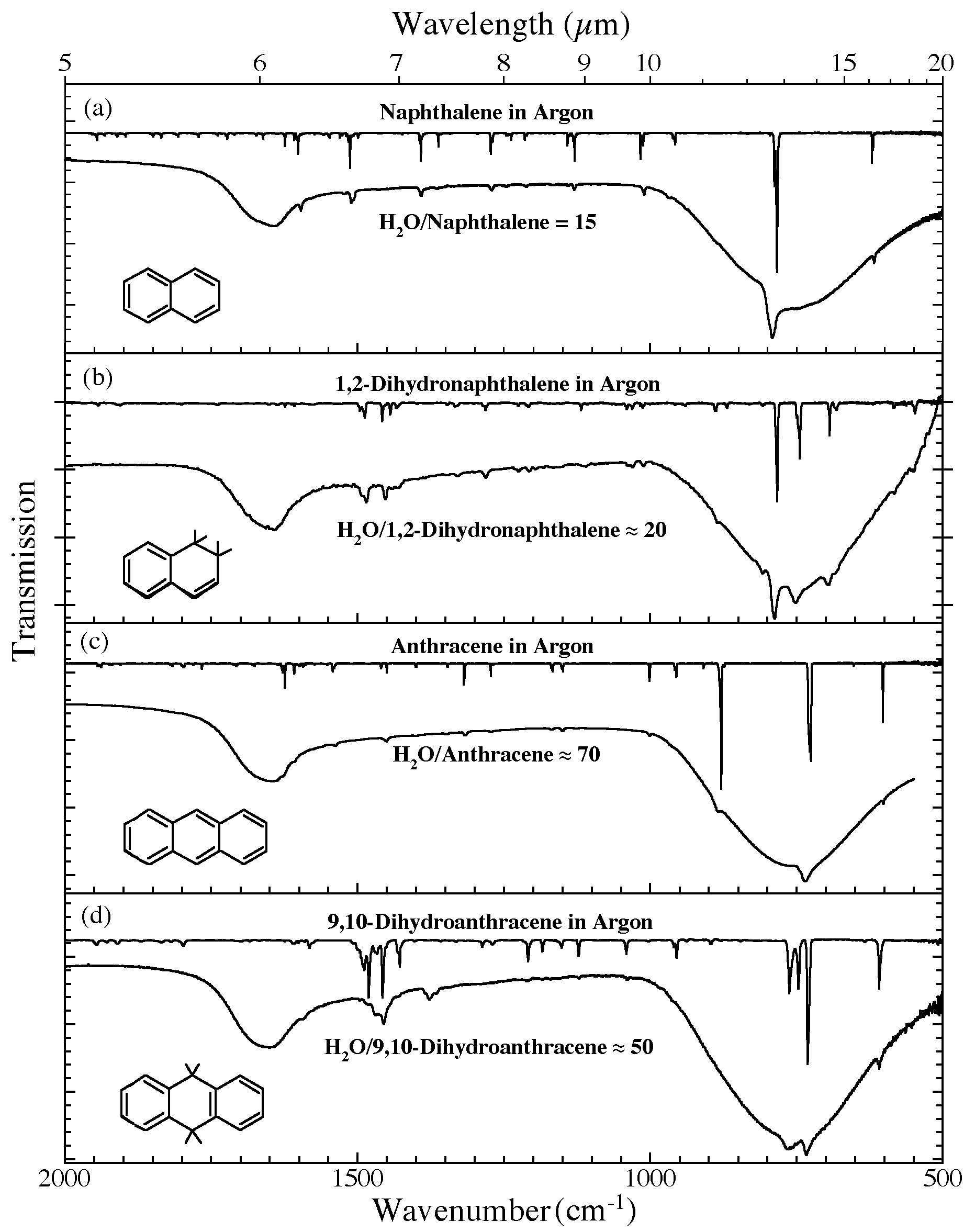
Figure 4. The 2000-500 cm-1 (5.0-20.0 microns) infrared spectra of (a) H2O/naphthalene ~ 15, (b) H2O/1,2-dihydronaphthalene ~ 20, (c) H2O/anthracene ~ 70, and (d) H2O/9,10-dihydroanthracene ~ 50. Each H2O/PAH spectrum is
compared to the spectrum of the relevant PAH isolated in an Ar matrix. The Ar matrix data for naphthalene and anthracene were taken from Hudgins & Sandford (1998a). The Ar matrix data for 1,2-dihydronaphthalene and 9,10-dihydroanthracene are taken from Bernstein et al. (2005). All
spectra were measured from samples at 15 K.
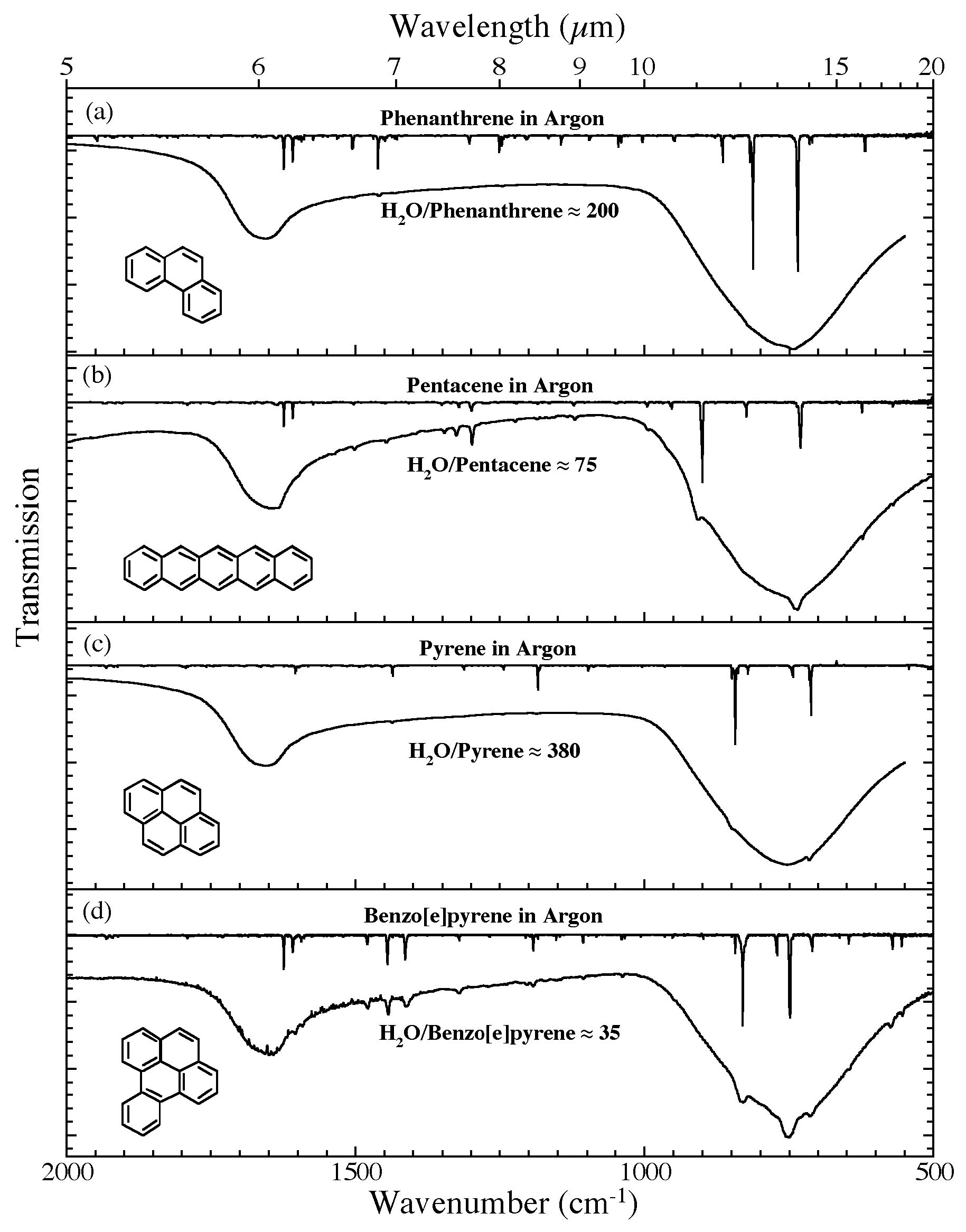
Figure 5. The 2000-500 cm-1 (5.0-20.0 microns) infrared spectra of (a) H2O/phenanthrene ~ 200, (b) H2O/pentacene ~ 75, (c) H2O/pyrene ~ 380, and (d) H2O/benzo[e]pyrene ~ 35. Each H2O/PAH spectrum is compared to the spectrum of the
relevant PAH isolated in an Ar matrix. The Ar matrix data for phenanthrene and pyrene are taken from Hudgins & Sandford (1998a); those for pentacene and benzo[e]pyrene are taken from Hudgins & Sandford (1998b).All spectra were measured from samples at 15 K.
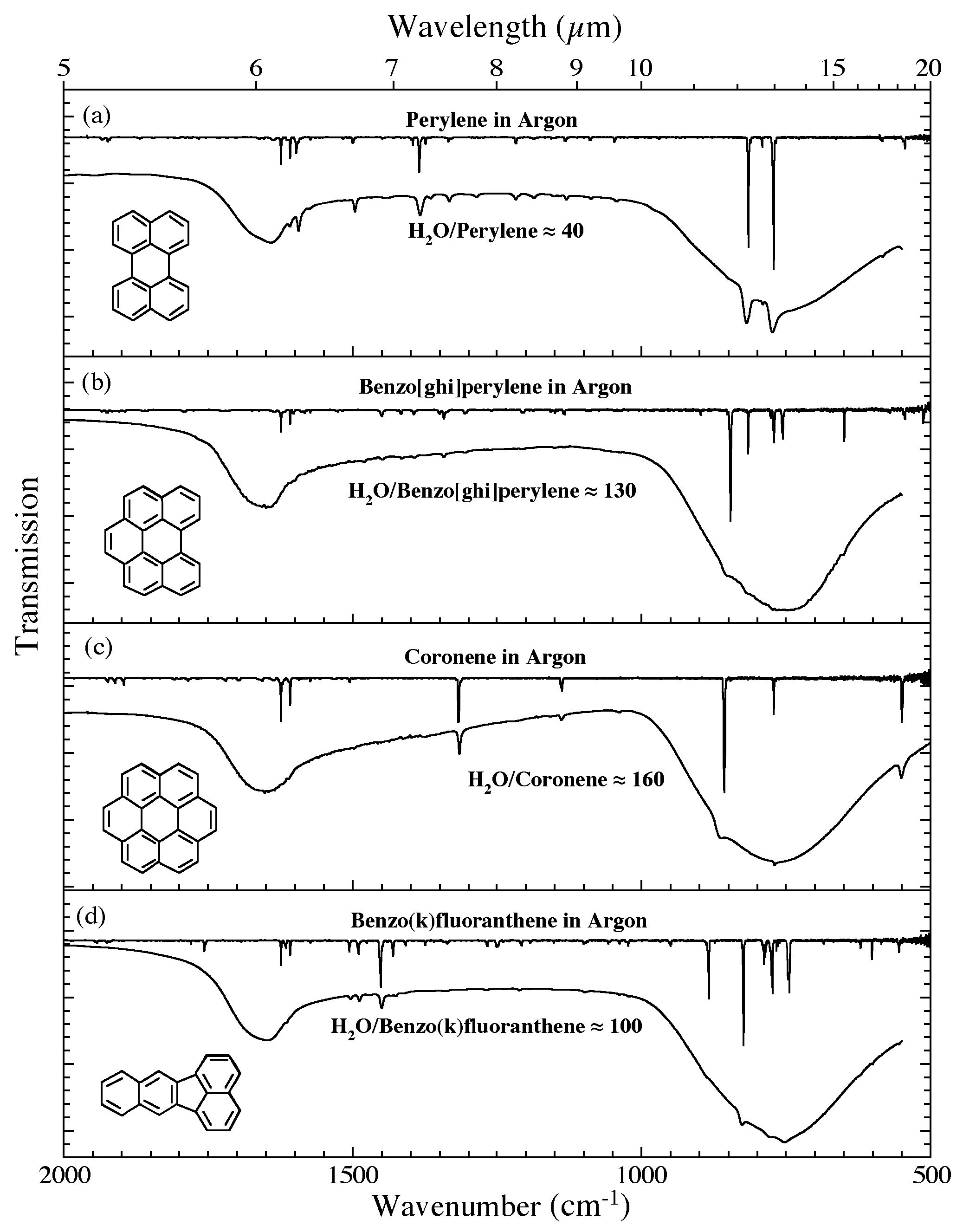
Figure 6. The 2000-500 cm-1 (5.0-20.0 microns) infrared spectra of (a) H2O/perylene ~ 40, (b) H2O/benzo[ghi]perylene ~ 130, (c) H2O/coronene ~ 160, and (d) H2O/benzo(k)fluoranthene ~ 100. Each H2O/PAH spectrum is
compared to the spectrum of the relevant PAH isolated in an Ar matrix. The Ar matrix data for perylene, benzo[ghi]perylene, and coronene are taken from Hudgins & Sandford (1998b); the spectrum of benzo(k)fluoranthene are taken from Hudgins & Sandford (1998c). All spectra were measured from samples at 15 K.
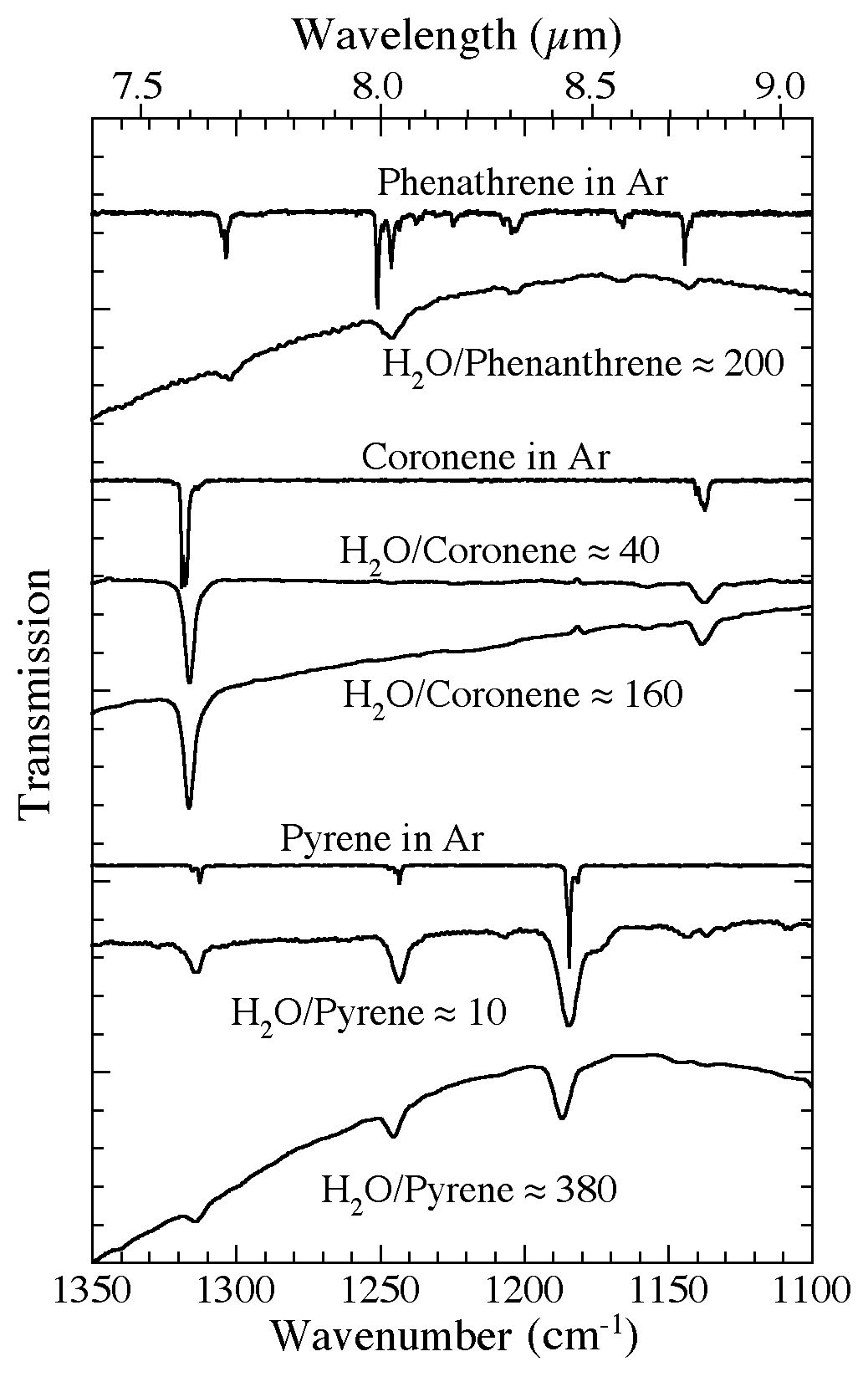
Figure 7. The 1350-1100 cm-1 (7.41-9.1 microns) infrared spectra of several PAHs frozen in Ar and in H2O-rich ices
at different concentrations. Compared to their spectra when isolated in Ar, the absorption bands of PAHs in H2O ices generally show increased widths, but only modest shifts in position and relative band strengths. The magnitude of these changes is largely independent of
concentration over the range considered here. All spectra were taken from samples deposited and maintained at 15 K.
References
Allamandola, L. J., Hudgins, D., and Sandford, S. A., "Modeling the Unidentified Infrared Emission with Combinations of Polycyclic Aromatic Hydrocarbons", Astrophys. J., 511, L115, 1999
Allamandola, L. J., Tielens, A. G. G. M., and Barker, J. R., "Interstellar polycyclic aromatic hydrocarbons - The infrared emission bands, the excitation/emission mechanism, and the astrophysical implications", Astrophys. J. Supp. Ser., 71, 733, 1989
Peeters, E., et al., "The rich 6 to 9 μm spectrum of interstellar PAHs", Astron. & Astrophys., 390, 1089, 2002
Sandford, S. A. and Allamadola, L. J., "Condensation and vaporization studies of CH3OH and NH3 ices: Major implications for astrochemistry", Astrophys. J., 417, 815, 1993
Sandford, S. A., "Interstellar processes leading to molecular deuterium enrichment and their detection", Planet. Space Sci., 50, 1145, 2002
Smith, R. G., Sellgren, K., and Tokunaga, A. T., "Absorption features in the 3 micron spectra of protostars", Astrophys. J. Part 1, 344, 413, 1989
Sellgren, K., et al., "A New 3.25 Micron Absorption Feature Toward Monoceros R2/IRS 3", Astrophys. J. Lett., 449, L69, 1995
Brooke, T. Y., Sellgren, K., and Geballe, T. R., "New 3 Micron Spectra of Young Stellar Objects with H2O Ice Bands", Astrophys. J., 517, 883, 1999
Chiar, J. E., et al., "The Composition and Distribution of Dust along the Line of Sight toward the Galactic Center", Astrophys. J., 537, 749, 2000
Bregman, J. D., Hayward, T. L., and Sloan, G. C., "Discovery of the 11.2 Micron Polycyclic Aromatic Hydrocarbon Band in Absorption toward Monoceros R2 IRS 3", Astrophys. J., 544, L75, 2000
Hudgins, D. M. and Allamandola, L. J., "Infrared Spectoscopy of Matrix-Isolated Polycyclic Aromatic Hydrocarbon Cations 2: The Members of the Thermodynamically Most Favorable Series through Coronene", J. Phys. Chem., 99, 3033, 1995a
Hudgins, D. M. and Allamandola, L. J., "Infrared Spectoscopy of Matrix-Isolated Polycyclic Aromatic Hydrocarbon Cations III: The Polyacenes Anthracene, Tetracene, and Pentacene", J. Phys. Chem., 99, 8978, 1995b
Szczepanski, J., Wehlburg, C., and Vala, M., "Vibrational and electronic spectra of matrix-isolated pentacene cations and anions", Chem. Phys. Lett., 232, 221, 1995
Hudgins, D. and Sandford, S.A., "Infrared Spectroscopy of Matrix-Isolated Polycyclic Aromatic Hydrocarbons 1. PAHs Containing 2 to 4 Rings", J. Phys. Chem. A, 102, 329, 1998a
Hudgins, D. M. and Sandford, S. A., "Infrared Spectroscopy of Matrix-Isolated Polycyclic Aromatic Hydrocarbons 2. PAHs Containing 5 or More Rings", J. Phys. Chem. A, 102, 344, 1998b
Hudgins, D. M. and Sandford, S. A., "Infrared Spectroscopy of Matrix-Isolated Polycyclic Aromatic Hydrocarbons 3. Fluoranthene and the Benzofluoranthenes", J. Phys. Chem. A, 102, 353, 1998c
Mattioda, A. L., et al., "Infrared Spectroscopy of Matrix-Isolated Polycyclic Aromatic Hydrocarbons, 6. Polycyclic Aromatic Nitrogen Heterocycles and their Cations", J. Phys. Chem. A, 107, 1486, 2003
Sandford, S. A., "The Inventory of Interstellar Materials Available for the Formation of the Solar System", Meteor. Planet. Sci, 31, 449, 1996
Ehrenfreund, P. and Charnley, S. B., "Organic Molecules in the Interstellar Medium, Comets, and Meteorites: A Voyage from Dark Clouds to the Early Earth", Ann. Rev. Astron. Astrophys., 38, 427, 2000
van Dishoeck, E. F., "ISO Spectroscopy of Gas and Dust: From Molecular Clouds to Protoplanetary Disks", Ann. Rev. Astron. Astrophys., 42, 119, 2004
Sandford, S. A., et al., "Laboratory studies of the infrared spectral properties of CO in astrophysical ices", Astrophys. J., 329, 498, 1988
Ehrenfreund, P., et al., "Ice segregation toward massive protostars", Astron. & Astrophys., 339, L17, 1998
Dartois, E., et al., "Carbon dioxide-methanol intermolecular complexes in interstellar grain mantles", Astron. & Astrophys., 351, 1066, 1999
Gerakines, P. A., et al., "Observations of Solid Carbon Dioxide in Molecular Clouds with the Infrared Space Observatory", Astrophys. J., 522, 357, 1999
Sandford, S. A., Bernstein, M. P., and Allamandola, L. J., "The mid-infrared laboratory spectra of naphthalene (C10H8) in solid H2O", Astrophys. J., 607, 346, 2004
The Mid-Infrared Absorption Spectra of Neutral Polycyclic Aromatic Hydrocarbons in Conditions Relevant to Dense Interstellar Clouds
Bernstein, M. P., Sandford, S. A., and Allamandola, L. J., "The Mid-Infrared Absorption Spectra of Neutral Polycyclic Aromatic Hydrocarbons in Conditions Relevant to Dense Interstellar Clouds", Astrophys. J. Supp. Ser., 161, 53, 2005
|